Molar mass of cac2o4 h2o – Embark on a captivating journey into the realm of chemistry as we delve into the fascinating world of molar mass, with a particular focus on the intriguing compound CaC2O4·H2O. This comprehensive guide will illuminate the concept, calculation, significance, and applications of molar mass, providing a clear and engaging exploration of this fundamental aspect of chemistry.
Get ready to unravel the mysteries of molar mass, understand its practical implications, and discover the secrets hidden within the molecular structure of CaC2O4·H2O. Let’s dive right in!
Definition of Molar Mass
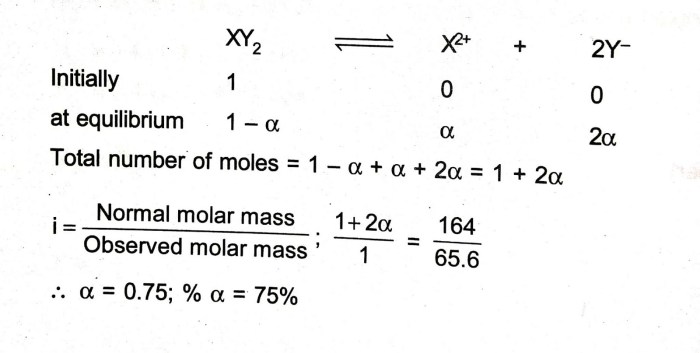
In chemistry, molar mass is a fundamental concept that measures the mass of a given substance relative to the mass of one mole of carbon-12 atoms. It represents the total mass of one mole of a compound or element, providing valuable insights into the quantitative composition of chemical substances.
The molar mass of a substance is expressed in grams per mole (g/mol). This unit represents the mass of one mole of the substance in grams. Understanding molar mass is crucial for various chemical calculations, such as determining the number of moles in a given mass of a substance, calculating the concentration of solutions, and predicting the mass of products in chemical reactions.
Calculating Molar Mass
Molar mass is a fundamental concept in chemistry. It provides the mass of one mole of a substance and serves as a crucial factor in various calculations.
Calculating molar mass involves a straightforward process. First, we identify the chemical formula of the substance. Next, we determine the atomic mass of each element in the formula using the periodic table. Finally, we multiply the atomic mass of each element by the number of atoms of that element in the formula and sum up these values to obtain the molar mass.
Formula for Calculating Molar Mass
The formula for calculating molar mass is:
Molar Mass = Σ (Atomic Mass of Each Element × Number of Atoms of That Element)
Molar Mass of CaC2O4·H2O
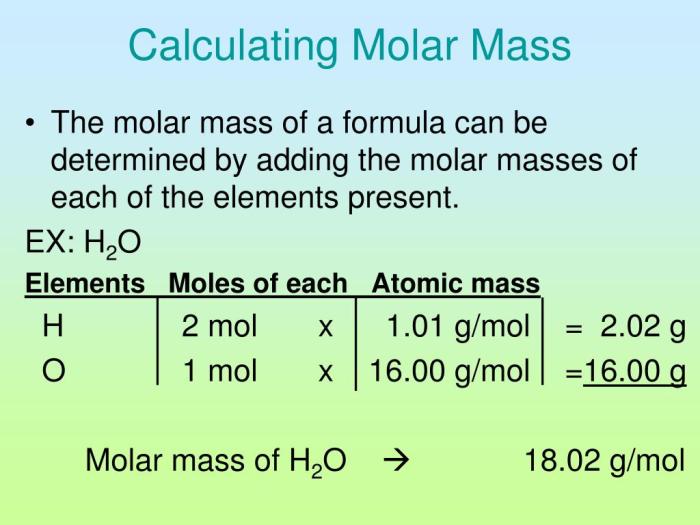
Calcium oxalate monohydrate (CaC2O4·H2O) is a compound commonly found in kidney stones. Its molar mass is crucial for various calculations and understanding its properties.
Determining the Molar Mass of CaC2O4·H2O
The molar mass of a compound is the sum of the atomic masses of all the atoms in its formula. For CaC2O4·H2O, the molar mass can be calculated as follows:
Atomic mass of Ca
40.08 g/mol
Atomic mass of C
12.01 g/mol
Atomic mass of O
16.00 g/mol
Atomic mass of H
1.01 g/molMolar mass of CaC2O4·H2O = (1 × 40.08 g/mol) + (2 × 12.01 g/mol) + (4 × 16.00 g/mol) + (2 × 1.01 g/mol) = 128.11 g/molTherefore, the molar mass of CaC2O4·H2O is 128.11 g/mol.
Significance of the Molar Mass Value
The molar mass value of CaC2O4·H2O is significant for several reasons:
- It provides the mass of one mole of the compound, which is essential for stoichiometric calculations in chemical reactions.
- It helps determine the number of moles present in a given mass of the compound.
- It is used in various physical and chemical calculations, such as density, solubility, and reaction yields.
Understanding the molar mass of CaC2O4·H2O is crucial for comprehending its properties and behavior in chemical reactions.
Applications of Molar Mass

Molar mass is a fundamental concept in chemistry and has numerous applications in various chemical calculations. It allows us to determine the mass of a given substance, predict the number of particles present, and understand the composition of compounds.
Determining Mass, Molar mass of cac2o4 h2o
Molar mass is used to determine the mass of a substance when its quantity is known. For instance, if we have 2 moles of sodium chloride (NaCl), we can calculate its mass using its molar mass (58.44 g/mol):
Mass = Number of moles × Molar mass
Mass = 2 mol × 58.44 g/mol = 116.88 g
Related Concepts

Molar mass is closely tied to other important chemical concepts, such as molecular weight and empirical formula.
Molar mass of CaC2O4·H2O, a compound used in analytical chemistry, is a topic often encountered in chemistry textbooks. Just like the enigmatic allure of her lips, which resemble the intricate beauty of copper wire as described by Stacy , the molar mass of CaC2O4·H2O, a seemingly complex concept, can be grasped with a touch of curiosity and a dash of determination.
Molecular weightis a term often used interchangeably with molar mass. However, there is a subtle difference between the two. Molecular weight refers to the mass of a single molecule, while molar mass refers to the mass of one mole of a substance, which is equal to the molecular weight multiplied by Avogadro’s number (6.022 x 10 23).
Empirical Formula
The empirical formulaof a compound represents the simplest whole-number ratio of atoms present in that compound. It does not provide information about the compound’s structure or the arrangement of atoms within the molecule. The empirical formula can be determined from the molar mass of the compound and the relative proportions of different elements present in it.
For example, the empirical formula of glucose (C 6H 12O 6) indicates that for every six carbon atoms, there are twelve hydrogen atoms and six oxygen atoms. However, the molecular formula of glucose is C 6H 12O 6, which means that the actual molecular structure contains one molecule of glucose.
Table of Values
Molar mass, a crucial concept in chemistry, is used to calculate various aspects of chemical reactions. To provide a comprehensive understanding, we present a table of molar masses for different substances, including CaC2O4·H2O.
Molar Masses of Substances
The molar mass of a substance represents the mass of one mole of that substance. The following table provides the molar masses of various substances:
| Substance | Molar Mass (g/mol) |
|---|---|
| CaC2O4·H2O | 128.11 |
| Sodium chloride (NaCl) | 58.44 |
| Water (H2O) | 18.02 |
| Carbon dioxide (CO2) | 44.01 |
| Glucose (C6H12O6) | 180.16 |
Examples
Here are some examples of calculations involving the molar mass of CaC2O4·H2O:
Calculating the Molar Mass of CaC2O4·H2O
To calculate the molar mass of CaC2O4·H2O, we add the atomic masses of each element in the compound multiplied by the number of atoms of that element:
Molar mass of CaC2O4·H2O = (1 × atomic mass of Ca) + (1 × atomic mass of C) + (2 × atomic mass of O) + (4 × atomic mass of H) + (1 × atomic mass of O)
Molar mass of CaC2O4·H2O = (1 × 40.08 g/mol) + (1 × 12.01 g/mol) + (2 × 16.00 g/mol) + (4 × 1.01 g/mol) + (1 × 16.00 g/mol)
Molar mass of CaC2O4·H2O = 128.09 g/mol
Calculating the Number of Moles of CaC2O4·H2O
To calculate the number of moles of CaC2O4·H2O in a given mass, we divide the mass by the molar mass:
Number of moles of CaC2O4·H2O = mass of CaC2O4·H2O (g) / molar mass of CaC2O4·H2O (g/mol)
For example, to calculate the number of moles of CaC2O4·H2O in 5.00 g of the compound:
Number of moles of CaC2O4·H2O = 5.00 g / 128.09 g/mol
Number of moles of CaC2O4·H2O = 0.0390 mol
Calculating the Mass of CaC2O4·H2O
To calculate the mass of CaC2O4·H2O required for a given number of moles, we multiply the number of moles by the molar mass:
Mass of CaC2O4·H2O (g) = number of moles of CaC2O4·H2O × molar mass of CaC2O4·H2O (g/mol)
For example, to calculate the mass of CaC2O4·H2O required for 0.100 mol of the compound:
Mass of CaC2O4·H2O (g) = 0.100 mol × 128.09 g/mol
Mass of CaC2O4·H2O (g) = 12.81 g
Q&A: Molar Mass Of Cac2o4 H2o
What is the significance of molar mass?
Molar mass is a crucial property that enables chemists to determine the mass of a specific number of molecules or atoms in a given substance. It serves as a bridge between the macroscopic and microscopic scales, allowing us to relate the mass of a bulk sample to the individual particles that constitute it.
How is the molar mass of CaC2O4·H2O calculated?
To calculate the molar mass of CaC2O4·H2O, we add the atomic masses of each element present in the compound. For CaC2O4·H2O, this involves adding the atomic masses of calcium (Ca), carbon (C), oxygen (O), and hydrogen (H), taking into account the number of atoms of each element in the formula.
What are some applications of molar mass in chemistry?
Molar mass finds numerous applications in chemistry, including determining the molecular weight of compounds, calculating the number of moles in a given mass of substance, and performing stoichiometric calculations in chemical reactions. It is a fundamental concept that underpins many essential calculations in the field of chemistry.